All News
-
Taipei Veterans General Hospital and NYCU Sign MOU
Professor Chun-Li Lin, Dean of the College of Biomedical Engineering and Director of the Medical Device Innovative and Translation Center, will lead the future collaboration between our university’s biomedical engineering field and the Medical Engineering Department of the Taipei Veterans General Hospital. This partnership will closely integrate clinical hospital resources, promote clinical trials and field cooperation, and build various medical data databases. Ultimately, the goal is to jointly develop medical devices that address high clinical needs.
Report LinkTaipei Veterans General Hospital and NYCU Sign MOUmore -

2023 Annual Innovation Medical Device Translational R&D Center Results Presentation
The upcoming conference will feature presentations from:
Director of the Plastics Industry Development Center, who will share insights on the challenges and shortcuts in the commercialization of innovative medical devices, focusing on maximizing cross-domain resource utilization.
Director of the Taipei Veterans General Hospital Department of Biomedical Engineering, who will discuss the paradigm shift in establishing smart hospitals, with a focus on innovation through smart medical device development.
Additionally, our center and team will share the latest developments in the application of nano-carrier drugs, wearable physiological sensing technologies, hearing and speech assistive systems, and brain imaging technologies.2023 Annual Innovation Medical Device Translational R&D Center Results Presentationmore -

10/6 Global Medical Device Market Opportunities and Regulatory Challenges Forum
The purpose of this forum is to provide business decision-makers with changes in regulations and market overviews in major markets such as the United States, the European Union, and China, as a relevant strategy to deal with these market changes.10/6 Global Medical Device Market Opportunities and Regulatory Challenges Forummore -

Join the Multi-Sensory Intelligent Healthcare Alliance!
Join the Multi-Sensory Intelligent Healthcare Alliance!
Enhance your expertise in this field and achieve expected outcomes! Want to learn more about multi-sensory medical devices, medical implants, regulations and practices for precision drug development, deep learning basics and applications, and diverse clinical practices, but not sure where to start?
Professional knowledge can be nurtured and solidified through academic learning, and students will develop cross-disciplinary expertise to foster innovation. This will allow fresh graduates to broaden their horizons and explore new perspectives. Take the opportunity to begin your 2024 journey!
Join our Information Session: Friday, April 21, 12:10 PM
Location: Biomedical Engineering Building, 1st Floor, Lecture Hall, Yangming Campus
Registration: https://reurl.cc/Dm3NL5 (Deadline: April 21, 9:00 AM)
Recommended Video: https://youtu.be/_QO31tr5SiQJoin the Multi-Sensory Intelligent Healthcare Alliance!more -

NYCU 2023 [ Medical Device Technician Training Program ]
NYCU 2023 [Medical Device Technician Training Program]
* Participants can apply for training hours certified by the Taiwan FDA (TFDA) upon course completion. *
Learn from top experts across academia, industry, and research organizations.
Gain practical insights into medical device regulatory applications and approval processes.
Enhance your company’s product registration efficiency, reduce costs, and improve market outcomes.
Course Dates: 3/31 – 7/14, 2023 (8 sessions, every Friday, 9:00 AM – 5:00 PM)
[ 03/31、04/14、04/28、05/19、05/26、06/30、07/07、07/14 ]
Format: In-person & Online
*Course Info: https://ppt.cc/f5URzx
*More Details: https://reurl.cc/GeVnVWNYCU 2023 [ Medical Device Technician Training Program ]more -

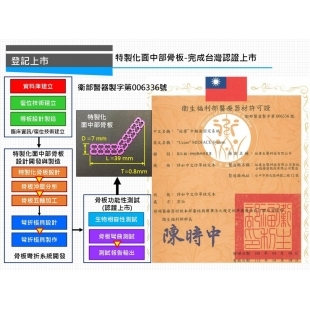
"New patient-specific MIDFACE Metal 3D Printed Bone Plate" Registered for Market Listing.
In clinical settings, fractures or defects in the midfacial bones can result from traumatic injuries, external impacts, or tumor-related diseases. Without proper treatment, these fractures may lead to issues such as facial asymmetry, misalignment of the bite, and double vision. Currently, the most effective treatment involves reconstructive surgery performed by plastic surgeons, using traditional microplates for midfacial fracture reduction and reconstruction. However, challenges such as poor positioning and unstable fixation still remain."New patient-specific MIDFACE Metal 3D Printed Bone Plate" Registered for Market Listing.more -

AI Smart Medical Devices and Sustainable Innovation Technology Trends Seminar
In recent years, the medical device industry has become a key emerging sector. The three-year COVID-19 pandemic has accelerated the rise of the medical device industry, making it a focal point for national industrial development. This seminar, jointly organized by the Plastics Center and National Yang Ming Chiao Tung University, will explore innovative technological applications and analyze the future trends of medical device development. Topics will include how medical device manufacturing can meet sustainable net-zero carbon emissions, the design and surface treatment technologies for invasive microcatheters, and the application of AI in various medical devices. The seminar aims to help industry players develop innovative medical products that are more aligned with future market demands and enhance industry competitiveness.AI Smart Medical Devices and Sustainable Innovation Technology Trends Seminarmore -

2022 New Generation International Youth Smart Healthcare Seminar and Presentation Conference
National Yang Ming Chiao Tung University and the National Science and Technology Council's Smart Medical Device Development and Testing Verification Service Platform (CIBDS Lab) have formed an alliance. They are inviting experts from the global smart medical field to organize an international seminar focused on cross-national, innovative, and biomedical visions. The goal is to create an academic exchange and industry development matchmaking platform. The seminar will also feature a press conference on industry-academia collaboration alliances. We sincerely invite all reporters to attend.
The International Biomedical Technology Research Alliance (B.I.T) is a group of members with biomedical engineering backgrounds who are passionate about smart healthcare. They initiate the i2i model (Initiative to Institution) to connect biomedical practice platforms, cross-disciplinary industry-government-academia collaboration, and promote biomedical innovation while cultivating interdisciplinary teams for biomedical entrepreneurship.
To build a communication bridge between domestic and international NGOs, B.I.T has gathered big data engineers, specialists, entrepreneurs, data scientists, designers, and other professionals who are enthusiastic about smart healthcare. By leveraging their resources and approaching the issues from various angles, they aim to solve real-world healthcare and medical problems.
The B.I.T Research Team focuses on helping healthcare professionals and collaborating with multiple medical alliances to inspire innovative solutions for smart healthcare. The team also trains and recruits globally competitive talents in smart healthcare, strengthens medical AI technologies, assists community members in publishing numerous international journal papers, and develops research capacity in smart healthcare. The ultimate goal is to bring biomedical industries to fruition and enhance the quality of medical care.
-----------------------------------------------------------------------------------------------------------------------------------
Organizer:BIT Research Alliance & CIBDS Lab
Guiding Organizations:National Science and Technology Council (NSTC)、NYCU
Date:2022/10/23 9:30-17:30 (GMT+8)
Location:NYCU Activity Center, Conference Room 2 (No. 155, Section 2, Linong Street, Beitou District, Taipei City)
Event Format:The event will be held both online and offline. A press release link will be provided after the event.
Media Registration Link:https://forms.gle/beM3DAzCr57Hwgv992022 New Generation International Youth Smart Healthcare Seminar and Presentation Conferencemore -

September to October "Smart Medical Device Regulations Course"
Latest Regulatory Course Sharing - Smart Medical Device Regulations Course
September to October "Smart Medical Device Regulations Course"more -

Biomedical Sensing and Speech Processing Technology Workshop
This seminar will explore the integration of three sensory technologies—auditory, speech, and visual—with AI, to deeply examine the professional knowledge and expertise that clinical personnel use in applying sensory interactions such as vision, hearing, and speech in medical diagnosis, treatment, and patient interactions within the field of smart medical devices. The aim is to enhance the value and functionality of the multi-sensory smart medical devices being developed.
If you are interested in hearing more experience sharing and participating in live interactions, we warmly invite you to register and join us!Biomedical Sensing and Speech Processing Technology Workshopmore -

Preparation of Medical Device Registration Documents and Procedure Management: Practical Guide for FDA Registration Submission
Smart Medical Devices Series: May 4, 2022 - Preparation of Medical Device Registration Documents and Procedure Management: Practical Guide for FDA Registration SubmissionPreparation of Medical Device Registration Documents and Procedure Management: Practical Guide for FDA Registration Submissionmore -

Applications of Artificial Intelligence in Innovative Medical Devices and Biomedical IoT
★Lecture Title: Applications of Artificial Intelligence in Innovative Medical Devices and Biomedical IoT
★Speaker: Mr. Chien-Sheng Liu, CEO of Lichuang Biomedical Technology Co., Ltd.
★Lecture Time: April 27 (Wednesday), 12:00 PM - 1:00 PM (20 min presentation / 10 min Q&A)
★Meeting Link: https://nycu.webex.com/nycu/j.php?MTID=m793f30ab4b8fae6b20118c15592fe8be
Applications of Artificial Intelligence in Innovative Medical Devices and Biomedical IoTmore